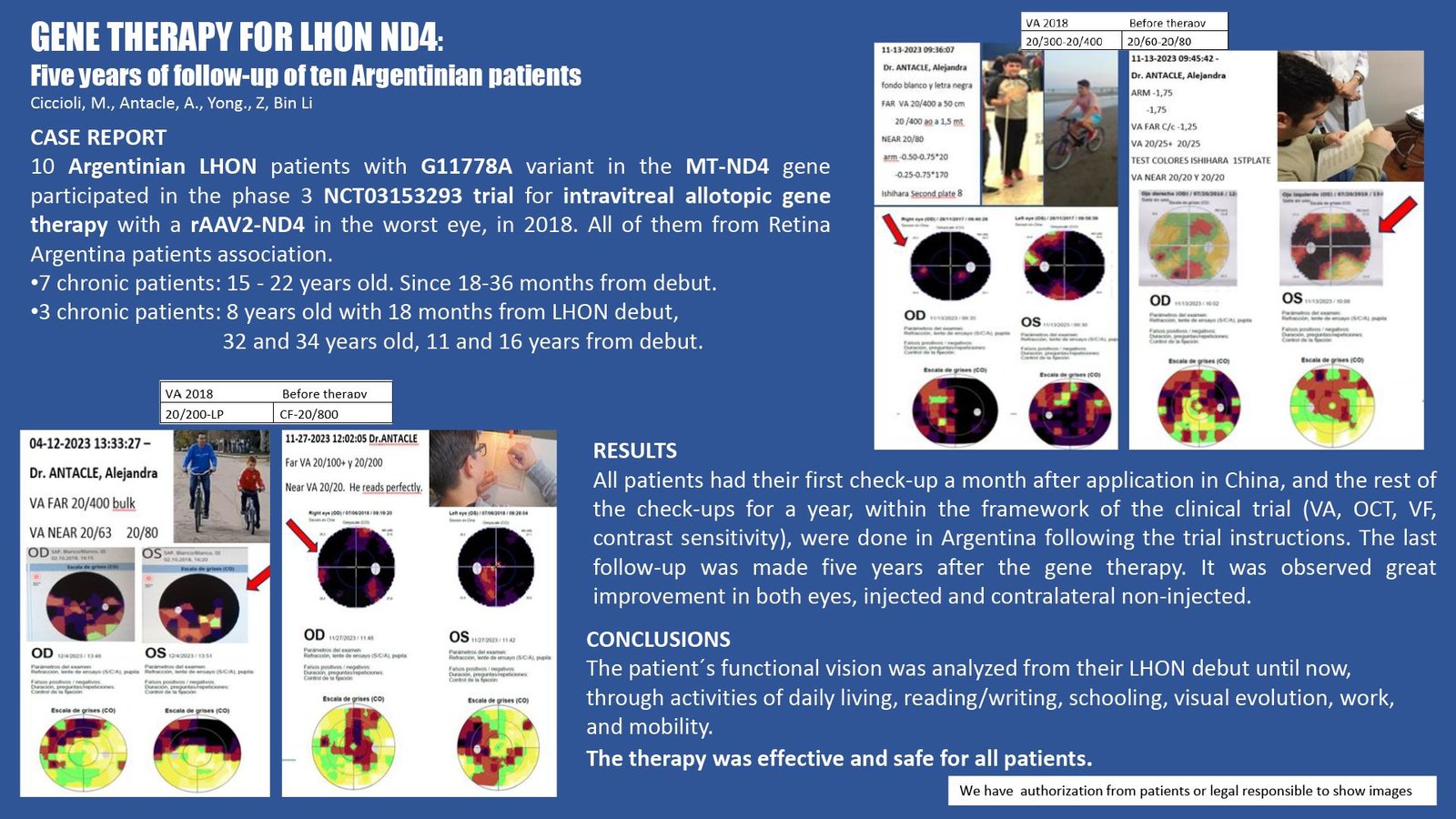
CASE REPORT

Gene therapy for LHON ND4: five years of follow-up of ten Argentine patients
PRESENTING AUTHOR
Marcela Ciccioli
-
M., Antacle,
-
A., Yong,
-
Z.Bin Li.,
-
Purpose:
Follow-up of patients who received gene therapy for LHON during Phase 3 clinical trial.
-
Case Report:
10 Argentine patients with LHON by G11778A in the MT-ND4 gene were selected to participate in phase 3 NCT03153293. The initial condition was during a chronic period. All patients received a single dose of rAAV2-ND4 only in the worst eye at the Shiyan Taie Hospital. They had their first check-up a month later in China and the rest of the check-ups for a year, within the clinical trial framework, were done in Argentina following the trial instructions. The last follow-up was made five years after the gene therapy observed great improvement in both eyes, injected and contralateral non-injected.
-
Discussion:
The evolution of parameters and safety controls were checked during the clinical trial and after for example visual acuity VA, optic coherence tomography OCT, visual fields VF, contrast sensitivity, and daily activities.
-
Conclusions:
The therapy was effective and safe for all patients.
The authors have no financial interests in any material discussed in this article. There are no conflicts of interest to disclose.